More Details of the January 22nd 2018 Chlorine Attack in Douma, Damascus
The following report was created in partnership with Syrians for Truth and Justice (STJ). The Arabic version of this report can be found here.

Image shows remnants of one of the rockets filled with a a chemical agent, reportedly chlorine, that fell on Douma on January 22, 2018 (Photo credit: STJ)
Preface
Syrian regular forces continued their military escalation in several towns and cities in the besieged Eastern Ghouta, as they launched the most violent military attacks on those areas using several types of weapons. On Monday morning, January 22, 2018, the residential neighbourhoods in the northwest region of Douma were subjected to an attack with a chemical agent described as chlorine gas by locals, and according to many testimonies obtained by Syrians for the Truth and Justice(STJ), several rockets loaded with chemicals believed to be chlorine gas fell in those neighbourhoods, suffocating 21 civilians including women and children.
The attack came in the context of the military campaign launched by the Syrian regular forces on whole cities and towns in Eastern Ghouta on November 15, 2017, following Ahrar al-Sham al-Islamiyya announced the battle “They Were Wronged” that began in three phases. The first phase began on November 14, 2017, when the fighting resulted in killing several soldiers of the Syrian regular troops, as well as Ahrar al-Sham al-Islamiyya controlled large sections of the Military Vehicles Management in Harasta. The second phase began on December 29, 2017, when fighting led Ahrar al-Sham al-Islamiyya to impose a siege on the Military Vehicles Management as well as controlling “al-Ajami neighbourhood” and the automated bakery in addition to al-Hadaeq neighbourhood that stretches along the road between Harasta and Arbin from the west side of the Military Vehicles Management. The third phase began on January 28, 2018, when Ahrar al-Sham al-Islamiyya detonated a tunnel for the regular forces within the Military Vehicles Management in Harasta, killing and injuring a number of the Syrian regular forces.
It is noteworthy that this attack was the second chemical attack on Eastern Ghouta since the beginning of 2018. On January 13, 2018, the area between both Harasta and Douma was also shelled with poisonous gases, injuring six civilians, including women and children, according to a report prepared earlier by STJ.
Details of the Incident
Nu’man Slick, who hails from Douma and who was near the strike location, spoke to STJ, saying:
“Given the intensity of the shelling on Douma, we took the cellars and shelters as our homes, as we no longer left them unless to serve our necessary needs. Once, while my family and I were in the vault, exactly at 5:30 am, I smelled a strange smell that seemed like chlorine that we use in houses; and instantly I rushed to awaken my children and I took them to a double-locked room. After that, I began to scream in order to awaken the people, and only minutes later, people started to run in every direction. There had been children who felt unconscious and were unable to breathe well; I tried to help them along with some people nearby as we put wet fabrics on their faces.”
In another testimony, Mohammed Oyoun, a doctor from Damascus Countryside Specialty Hospital, said that the hospital began to receive suffocation cases at around 5:45 am and there was about 21 cases of suffocation, with symptoms similar to those of toxic chlorine gas::
“The hospitals received 21 suffocation cases, including 8 men, 6 women and 7 children, with a child who is less than three months. The injured showed the following symptoms: dyspnea, a cough and a neurological irritation, in addition, their clothes smelled of chlorine. We were able to cure most of the cases through the spray with oxygen and bronchodilators. Many cases were directly cured, whereas many remained under surveillance for 24 hours; thank God, no casualties were recorded because the concentration of chlorine substance was not so great in the rockets pounded by the regime as I believe, in addition that the sunrise had softened many of the infected numbers because the sunrise had dissipated the poisonous gas.”
Videos filmed after the attack at a local hospital show many of the victims receiving treatment, including 7 children, and 7 adult males, consistent with reports from the Damascus Countryside Speciality Hospital.

Image shows some of the child victims of the on Douma on January 22, 2018 (Photo credit: Activists from Douma)
On January 22, 2018, the Directorate of Health in Damascus and its countryside issued a statement talking about the shelling of Douma with rockets loaded with poisonous gases, which caused suffocation among civilians. It also indicated that it was not the first time that densely populated areas in Douma are subjected to suffocation cases due to exposure to toxic gases, because the city had already been subjected to a similar attack on January13, 2018.

Image shows the statement issued by the Directorate of Health in Damascus and its countryside on January 22, 2018 (Photo credit: the Directorate of Health in Damascus and its countryside)
On the same day, the local council of the city of Douma condemned the attack against populated areas and stressed that the city had been subjected to a similar attack nine days earlier. The statement appealed to international organizations to assume their responsibilities in protecting civilians.

Image of the statement issued by the Local Council of Douma on January 22, 2018 (Photo credit: The Local Council of Douma)
Mohammed Al-Shami, alias, an armed Syrian opposition member who was near the strike location, spoke to STJ, saying:
“The north-west area within the stadium located in Douma, along with al-Manfoush neighbourhood had been targeted with 9 rockets loaded with poisonous gases. Sound of the rocket launch and the way it fell indicated that it was a Katyusha rocket, which was a Russian-made propellant charge that the regime had modified by supplying it with a homemade cylinder and the head was fitted with homemade detonators, leaking poisonous gas immediately after hitting the ground. It should be mentioned that five rockets had fallen directly on residential areas, whereas the four left had fallen in the agricultural areas adjacent to Douma.”

Image shows remnants of one of the rockets loaded with a chemical agent used in Douma on January 22, 2018.
(Photo credit: STJ)

Another image shows several modified rockets that had been used in the January 22 chemical attack, 2018 (Photo credit: STJ)
The munitions used in the attack were modified Iranian 107mm artillery rockets, with the explosive warhead replaced with a larger pressurized gas cylinder. Additional tail fins have been added to the rocket to provide additional stability.
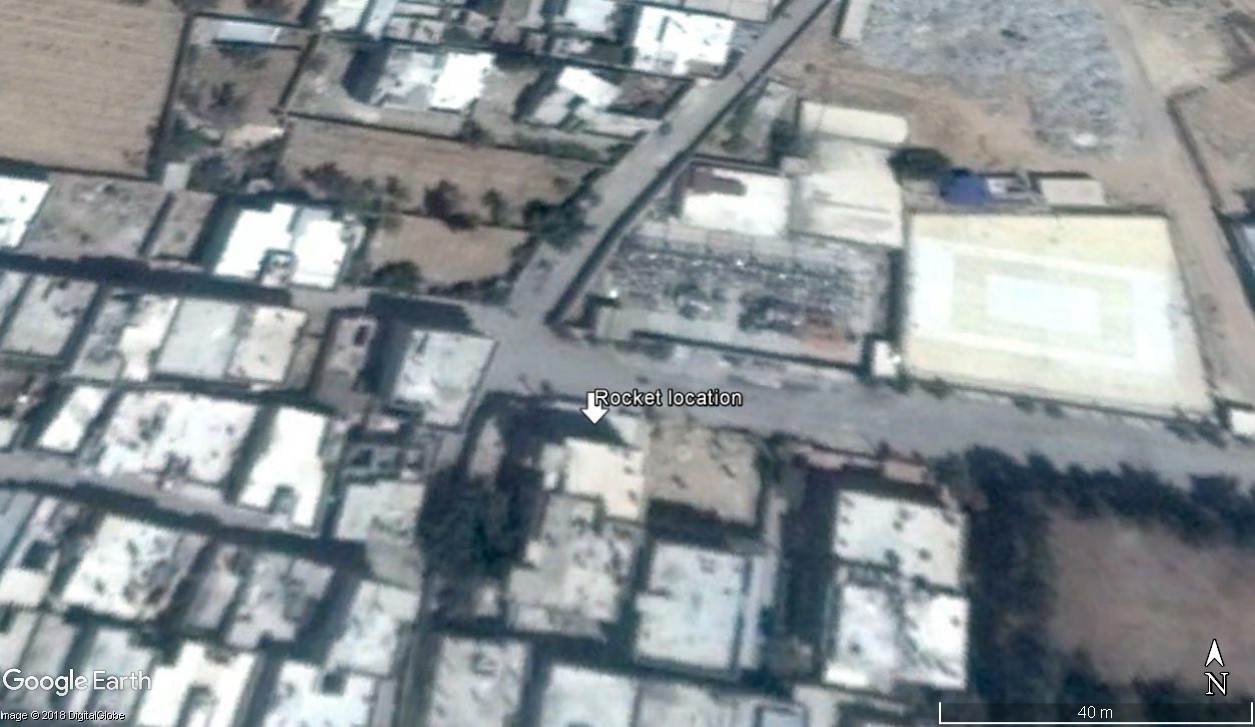
Jamal al-Hasan, an activist from Douma who was the first one to reach the impact site, told STJ that one of the rockets loaded with poisonous gases was found embedded in the dirt, and 15 m from its strike location, another rocket was found fallen on a house. The rocket described as found fallen on a house may refer to one rocket it was possible to geolocate, featured in several photographs:

One of the munitions used photographed on the roof of a building (Photo credit: Media activist from Douma (During an interview with STJ’s reporter))

The same munition as above, moved to the ground (Photo: Media activist from Douma (During an interview with STJ’s reporter))
The location the photograph on the roof was taken is 33.572149, 36.387439, on the roof a one storey structure running west to east on the north side of a larger apartment building. In addition to the geolocation of the photographs, the location was also confirmed by locals who took photographs of the munition.
Jamal al-Hasan noted that the rocket was carrying a white material similar to ice on its surface. In this regard, he continued:
“With the onset of the sunrise, this white material began to fade gradually, and once I attempted to touch it, it was very fragile, which caused me a severe headache lasted until the evening. Even the animals have not been spared the effect of this poisonous gas, as I saw a dead cat near the impact site.”

A photograph published by Bassam Khabieh on Facebook on January 22nd showing a dead cat close to one of the impact sites (Photo credit : Bassam Khabieh)

A photograph published by Bassam Khabieh on Facebook on January 22nd showing the munitions used with frost on the warhead (Photo credit: Bassam Khabieh)
The white material described by Jamal al-Hasan is likely frost formed as the pressurized gas cylinder depressurized rapidly. Analysis of images of the munitions provided by locals shows the munition would have had a pressure valve on the front, that would have been used to fill the warhead with pressurized gas.

One of the munitions used in the January 22nd attack decorated by the Syrian artist Akram Abo Alfoz with the pressure valve used for filling the munition visible of the front of the warhead (Photo credit : Akram Abo Alfoz)
The two objects in the rear of the warhead appear to be pressure relief valves, although the exact function is yet to be established. An extensive gallery of images showing the munitions in detail can be found here.
Bellingcat’s research for this publication was supported by PAX for Peace.