Amines and Sarin – Hexamine, Isopropylamine, and the Rest…
(Revised edition, May 2017)
One subject that keeps coming up in ongoing debates about Sarin chemical attack in Syria from 2013 to date is the nature, role, and presence of possible additives to Sarin. A molecule of Sarin is pretty much the same molecule however and wherever it was made. However, even in the best laboratory settings you don’t ever get truly pure Sarin. Relatively pure Sarin, with some additives, is the best for long term storage and the engineering needed to get pure Sarin is usually a level of expense and difficulty greater than anyone wants to expend, or is even capable of doing. The “other stuff” that ends up in Sarin – byproducts, impurities, contaminants, residue, additives and whatnot is often what gives away the most useful information. The purpose of this article is to explain in general terms the roles amines can play in the manufacture or storage of nerve agents.
The issue of amines in Sarin has been a peripheral part of the Syria debate since shortly after the Aug 2013 attacks. First, there was the discovery of Hexamethylenetetramine, commonly known as hexamine, in a large number of the field samples taken at the August 21st impact sites examined by the UN/OPCW team. Second, there were various amines reported to be in the inventory of the Syrian government, including hexamine and isopropylamine. These chemicals are on the inventory lists in the OPCW’s released document because these amines were judged by somebody, somewhere, to be part of the Syrian government’s chemical weapons production program. Whether that was an assessment by the OPCW, an admission by the Syrian government, or both, remains to be seen. However, a thorough discussion of the history and role of amines in the production of nerve agents is necessary in order to understand the Syrian situation.
What is an amine?
Amines are a chemical family of compounds. In broad terms, amines are chemicals with a basic nitrogen atom with a “lone pair” – a valence electron pair which is not shared with another atom. Amine compounds contain one or more nitrogens attached to carbon or hydrogen. That leaves an unbonded electron pair on the nitrogen atom, one chemical definition of a base. Those electrons will attract and bond with compounds or atoms that are deficient in electrons, like H+ (hydrogen ion, proton), which is characteristic of common acids. H+ is missing a pair of electrons. These definitions of acid and base are those in general use in most elementary chemistry textbooks. There are many amines in widespread use in for scientific, medical, and industrial uses.
Why would you use amines in Sarin production?
While amines have a role to play in some other nerve agents, the nerve agent Sarin was involved in the a number of chemical weapons attack in Syria. For this reason, this article focuses on Sarin. However, amines have uses in other nerve agents as well, so where applicable, this will be noted.
The production of Sarin can be accomplished by a number of different production paths, about twenty of which are known to me. My own ethics and conscience, as well as various legal and regulatory issues, prevent me from providing an exhaustive listing of these various production paths. However, I can summarize by saying that all of the production paths end with either hydrogen chloride (HCl) or hydrogen fluoride (HF) as a by-product. The second to-last step of all of the production pathways is one of two reactions:
1 mol DF + 1 mol Isopropyl Alcohol = 1 mol Sarin + 1 mol HF
1 mol DF + 1 mol DC + 2 mols Isopropyl Alcohol = 2 mols Sarin + 2 Mols HCl
The resulting acid is bad for a number of reasons. HF is worse in general terms than HCl, but both are bad for the following reasons:
- The production of acid in an exothermic (heat-creating) reaction, such as the reactions that produce Sarin, can rapidly cause an unsafe situation in the production lab or factory. A sudden arrival of a hot corrosive gas requires special chemical engineering measures to handle and can destroy equipment or kill people. Earlier in my career, I interviewed technicians who claimed that the production of HF in US binary shells (the M687 project) was enough to cause those shells to explode in flight on their way to the target, an obvious design flaw. These test reports remain secret, so I cannot confirm this.
- The acid by-product can make the Sarin-acid mix unsafe to handle – for example, acids can easily destroy most of the standard types of protective clothing and equipment that a soldier or technician would wear. HF in vapour form will not be stopped by military chemical protective equipment, for example. There is anecdotal information from the Iran-Iraq war that Iraqi chemical soldiers suffered injury or even death from combining the binary components, as their Soviet-designed mask and suits, while adequate for protection from Sarin, did not resist the corrosive effects of HF.
- The resulting Sarin-Acid cocktail will have a very short shelf-life. Sarin does not last very long in either high or low pH levels. The shelf-life of well-made Sarin can be very long under correct conditions. However, the shelf-life of Sarin with residual acid can be greatly reduced. Saddam Hussein’s Sarin had a shelf life of only a few months, in part or in whole due to residual acid.
- The acid will corrode weapons and storage containers. By weight, Sarin produced from the DF + Isopropyl method will have 140 g of HF for every kilogram of Sarin produced. This is a level of hydrofluoric acid that will be very corrosive to nearly any conceivable storage container or munition. Indeed, such a corrosive mix may, over time, interfere with fuzes or bursting charges, or leak out of the munition. Saddam Hussein’s Sarin-filled rockets had serious corrosion problems, largely due to acids.
- Mechanical and thermal methods of removing the residual acids are very difficult to achieve. The US and the USSR had to devote an extremely elaborate and expensive engineering effort to solving this problem, and it resulted in a very large infrastructure. The US and USSR had developed different methods for acid removal, but both are very expensive and require significant effort as well as specially designed apparatus.
Amines, in general, are useful as acid scavengers. Depending on the structure of the amine, the amine molecule will latch on to acid molecules, such as HCl or HF. In layman’s terms, the “lone pairs” in amines are, in effect, like parking spots for acid molecules. This means that if you add an amine in at the correct step of the manufacturing process for Sarin (or Soman) a lot of acid can be removed from the mix. Amines have a long history as acid reducers and anti-corrosion additives for basically the same reason.
Various Amines in Nerve Agents
Some commentators have recently claimed that only isopropylamine is used in Sarin production. This is patently untrue, as any basic research on the subject will indicate. None of the thousands of tons of US unitary Sarin produced at Rocky Mountain Arsenal use isopropylamine for acid scavenging. The assertion in various documents that this is the “standard US method” are misleading.
Numerous amines have been experimented with in nerve agent production, for various reasons. As the open literature on nerve agent production represents only a tiny fraction of the total body of knowledge on the subject, I cannot claim by any stretch that the following list of chemicals is comprehensive.
Tributylamine (CAS 102-82-9): Tributylamine was used as an anti-corrosion inhibitor in Sarin produced by the US Army at Rocky Mountain Arsenal in the 1950s. It was added after Sarin was produced and the vast majority of the acid removed by the US’s secret refining method. Pure Sarin has a slow corrosive effect on steel over time, and any residual acid exacerbates this problem. Tributylamine was needed as an additive to prevent corrosion in the bomblets for the Honest John missile and was added to much of the Sarin arsenal in order to prevent corrosion in steel containers or munitions. Unitary Sarin in the US inventory had an average tributylamine content of 1.95%.
It appears that tributylamine was used in quantity by the Libyan chemical weapons program, as quantities of it were destroyed as part of the chemical demilitarisation effort there.
Triethylamine (CAS 121-44-8): This substance is used in many commercial acid reduction processes. A declassified document from Porton Down in 1956, now available online through the US government, discusses that triethylamine was used in the United Kingdom as an acid-reducer in Sarin. This document indicates that triethylamine was considered the standard acid-reducing additive in UK Sarin. The same document also notes certain problems with triethylamine. UK-produced Sarin had shelf-life problems and, based on the production rate of the UK’s pilot plant at Nancekuke, Cornwall, which produced Sarin at a small rate to replace Sarin that had degraded, it seems that UK Sarin had perhaps a shelf life of a year or two. This is only an estimate, however.
Iraq is known (through revelations from UNSCOM) to have attempted to use triethylamine as an acide scavenger and additive. It is also known that the Aum Shinrikyo cult in Japan experimented with triethylamine as a Sarin additive, but without success. In Syria, 30 tons of triethylamine were declared in Syria’s OPCW declaration.
Isopropylamine: (CAS 75-31-0): Isopropylamine is another useful acid scavenging amine, with a history of use in nerve agents. It’s primary documented use was in the US M687 binary chemical artillery shell. (See my reference on binaries for greater discussion of binary Sarin.) This substance is highly soluble in isopropyl alcohol, giving it utility in binary formulations wherein the materials are mixed inside the weapon system. The prime example of this is the US M687 155mm binary Sarin artillery shell. One component of the M687 binary shell was a canister containing a cocktail of isopropyl alcohol and isopropylamine. It would appear that isopropylamine is actually less efficient at scavenging acid than many other amine compounds, and I can find no use for it outside of binary applications. The Syrian OPCW declaration includes 40 tons of this chemical.
N, N-diethylaniline: (CAS 91-66-7) The Aum Shinrikyo cult used N, N-diethylaniline in their Sarin formulation. This was apparently done after their experiments with triethylamine failed. The Aum cult acquired 50 tons of this chemical. The overall effectiveness of this approach is not known, as we have very little idea about the overall shelf-life or corrosive properties of the Aum group’s Sarin.
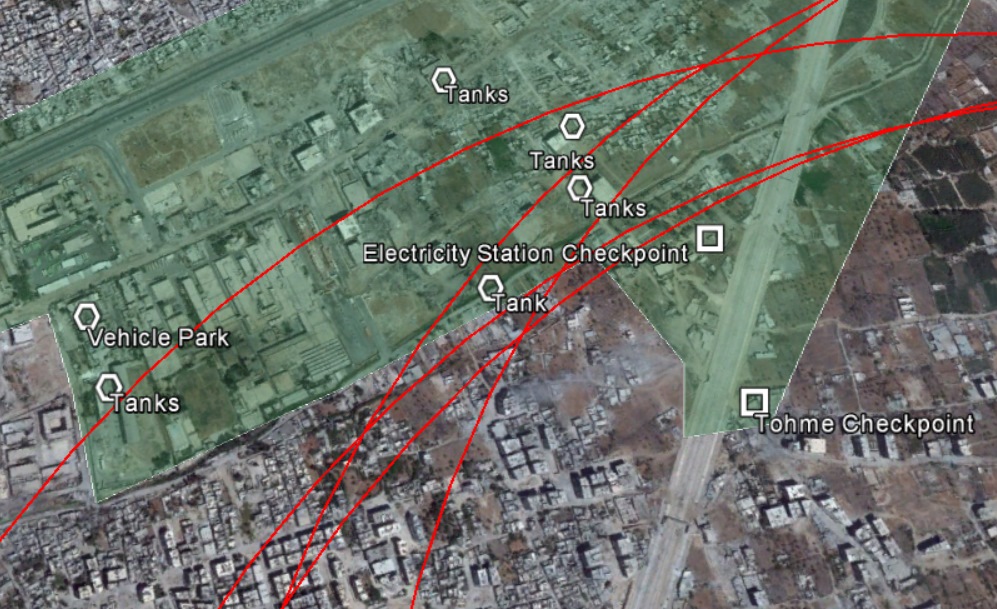
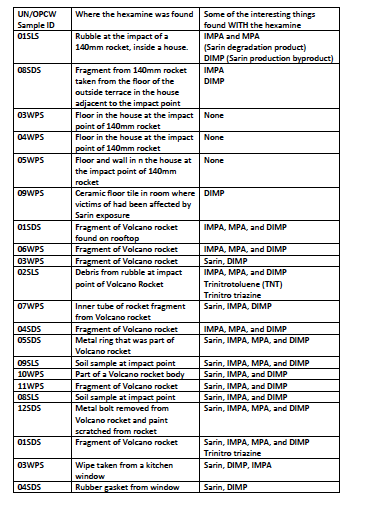
Hexamine: (CAS 100-97-0) Up until the war in Syria, the chemical hexamine had not been noted in the manufacture of or post-manufacture storage and handling of nerve agents. It was not considered a precursor or relevant chemical in any of the schedules of the Chemical Weapons Convention (CWC) nor was trade in hexamine of particular interest to people studying chemical proliferation. However, hexamine appears to have been incorporated into the Sarin produced and used by the Syrian government in at least three different Sarin incidents: April 2013, August 2013, and April 2017. In all three incidents, hexamine was found in environmental samples. In the case of the April 2013 attack, the French government obtained an intact grenade-type device which contained Sarin and hexamine. The joint UN/OPCW report in late 2013 provides a high degree of detail about the various places in which hexamine was found in the environmental samples from the Aug 2013 Ghouta incident. It should be noted that the report uses hexamine’s alternative name hexamethylenetetramine. French intelligence services report that hexamine was also found at the site of the April 2017 attack, in the same report referenced above.
In the UN/OPCW report, hexamine was found in a wide number of places in and around the Moadamiyah and Zamalka impact zones, wherein rockets containing Sarin were used to kill over 1000 people. I have produced this table to summarise the locations where hexamine was found, and what it was found alongside of.
After the Aug 2013 attack, Syria signed he CWC and allowed the OPCW to dismantle and dispose of its declared inventory of hardware, weapons, and chemicals. The declared inventory of precursor chemicals given to the OPCW show a substantial stockpile hexamine. 80 tons of hexamine were declared in the inventory, a quantity indicative of industrial-scale use. As it was not a scheduled chemical, there would have been no reason nor any requirement to disclose this stockpile unless it was part of the Syrian chemical weapons programme.
The role of hexamine as an acid scavenger in the production of Sarin was alluded to by members of the UN/OPCW mission that went to Syria in 2013 in a briefing to the press in December 2013. A number of references (see references 1, 2, and 3 below) point to the fact that a molecule of hexamine can bind with up to four molecules of HF. A patent from the Soviet Union points to the use of hexamine to remove HF from gas. Much comment has been made in various comments and documents about the solubility of hexamine in isopropyl alcohol. This is basically a red herring. Dissolving hexamine in isopropyl alcohol makes engineering sense if you are trying to make a binary weapon that mixes in flight (like the US M687). However, none of the weapon systems used in Sarin delivery to date in Syria are such a system. Use of hexamine by some other means, such as a slurry of finely powdered hexamine, have already been alluded to in comments by at least one chemist in the comments section of other Bellingcat posts. To date, no viable technical objection to the use of hexamine as an acid scavenger has survived scrutiny. And we are left with the very appropriate question – if it cannot be done, how is it that hexamine is all over the environmental samples AND was in the declared inventory of the Syrian CW programme. Several commentators have pointed to the lack of specific literature stating this particular use in Sarin. However, many aspects of manufacturing modern chemical warfare agents, particularly nerve agents, are not widely documented in open literature for understandable reasons. This should not be taken as a reason that it couldn’t be done.
There are other uses for hexamine, and some of these uses have been posited as alternative explanations for its presence in the environmental samples. It is used as a medicine for treating urinary tract infections, in some types of medical laboratory procedures, as a food additive, as a heating fuel (tablets of hexamine have been used as a cooking fuel in many militaries), as an anti-corrosion agent (such as an additive to paint), and for the manufacture of various kinds of explosives. Several of these bear additional comment.
Paint Additive: Theoretically, hexamine could have been added to paint as a corrosion preventer. This might have been a viable hypothesis if the environmental evidence was one or two fragments of a device and nothing else. Indeed, one cannot rule out (on the basis of evidence in the public domain) that hexamine may have been added to the paint on the ordnance used. Hexamine was found in a lot of places that seem to not have any paint, but also have either Sarin or its distinctive hallmarks. However, the quantities would be quite small, and certainly not enough to account for an 80 ton inventory. Furthermore, Sarin mixed with hexamine appears to have been found in the intact grenade-type device recovered by the French.
Cooking Fuel: Use as cooking fuel could, theoretically, account for a small portion of the samples, particularly debris from inside buildings. However, cooking fuel does not account for its presence in a crater in the road, or soil samples from Ghouta, or on the residue of different devices from three separate incidents.
Explosive manufacture: Hexamine is used to manufacture the explosive RDX, which is, in turn, incorporated into other types of high explosive, for example C-4. Some commenters have latched onto this as an explanation for the prevalence of hexamine in environmental samples. Discussing this with chemists as well as EOD technicians who do post-blast investigations, the easy way to say this is the Hexamine goes into the explosive as an ingredient, but it doesn’t come out as a byproduct. The chemistry does not appear to work that way, as explained in references 4 through 7 below. I can find no reference to hexamine as a decomposition product or residue relevant to explosive incidents, having conferred with several experienced post-blast investigators. (Indeed, the author would be happy to hear from one who has found it, in the cause of thoroughness. Please contact me.) Hexamine left over from the production of RDX is another proposed theory, but established methods (example here) wash away the leftover hexamine for re-use, and it would appear terribly wasteful and inefficient for a modern manufacturer to leave excess hexamine in their RDX, reducing the purity of the RDX. Furthermore, has it been established that RDX or RDX-based explosives were the disseminating explosives in these incidents? This is certainly plausible but I do not see where this has been clearly established. Indeed, one of the environmental samples showed TNT, not an RDX-based explosive. Finally, hexamine is flammable. If explosively disseminated some of it would surely combust. Signs of this are not in evidence. Finally, we get back to the 80 tons declared to the OPCW. It was declared as a component of the chemical weapon programme, not as a component in explosives manufacture, which is beyond OPCW’s remit.
Other Relevant Appearance of Amines in Nerve Agents:
Thickening agents: At least one declassified study refers to the use of various amine compounds in order to thicken G- and V- series nerve agents.
Decontamination: Not every amine is useful in the presence of a chemical warfare agent. Some can be quite destructive to some chemical warfare agent substances, some on their own, others in solution, e.g. dissolved in water or another solvent. The use of amines for decontamination of chemical warfare agents, has been well established in available literature for some time. For many years, the US military used a substance known as DS-2, which contained 70% diethylenetriamine. Monomethylamine, mixed with water, is a very good decontamination agent for G-series nerve agents. The older versions of the US Army’s decontamination manual FM 3-5 refers to dichloramine and hexachloramelamine as possible decontaminants for Sulfur Mustard. Isopropanolamine was investigated as a possible decontaminant. Numerous other amines may have utility in this regard.
Byproducts: Diisopropylamine is a commonly found impurity in VX. It is a possible decomposition product after decontamination of VX by decontamination involving hypochlorites. See ref 9.
Pyridostigmine: The drug pyridostigmine is a quaternary amine. It has some applications as a pre-treatment before human exposure to nerve agents, to improve the efficacy of oxime-based antidotes. It is of great value in protection against the nerve agent Soman, but of dubious value for protection against other nerve agents.
The author would like to thank the commentator “DDTea” for the references, which are previously published in comments sections for other Bellingcat posts.
References
- Ennan, A. A.; Brazovskaya, O. M.; Chopotarev, A. N. Products of the reaction between hydrogen fluoride and hexamethylenetetramine. Zhurnal Obshchei Khimii, 1975, Vol. 45, Issue 3, p. 706.
- Ennan, A. A.; Chobotarev, A. N.; Brazovskaya, O. M. “Hydrofluoric acid-hexamethylenetetramine-water system,” Zhurnal Neorganicheskoi Khimii, 1975, Vol 20, Issue 3, pp. 786-790
- Ennan, A. A.; Lapshin, V. A.; Brazovskaya, O. M.; Grishuk, N. S.; Mikhailovina, S. K. “Corrosion of steels in aqueous solutions of hydrogen fluoride containing urotropine,” Izvestiya Vysshikh Uchebnykh Zavedenii, Khimiya i Khimicheskaya Tekhnologiya, 1975, Vol.18, Issue 5, p. 840.
- J. D. Cosgrove and A. J. Owen. “The Thermal Decomposition of 1,3,5 Trinitro Hexahydro 1,3,5 Triazine (RDX)-Part 1: The Products and Physical Parameters” Combustion and Flame, Vol 22, Issue 1, Feb 1974, pp. 13-18
- A.C. T. van Duin, J. Oxgaard, and W.A. Goddard III. “Thermal decomposition of RDX from reactive molecular dynamics” J. Chem. Phys. 122, 054502 (2005).
- T.R. Botcher and C. A. Wight. “Explosive Thermal Decomposition Mechanism of RDX.” J. Phys. Chem. 1994,98, 5441-5444 .
- R. Behrens. “Thermal Decomposition of HMX and RDX: Decomposition Processes and Mechanisms Based on STMBMS and TOF of Velocity-Spectra Measurements” Chemistry and Physics of Energetic Materials
Volume 309 of the series NATO ASI Series pp 347-368 - Munro NB, Talmage SS, Griffin GD, et al. The sources, fate, and toxicity of chemical warfare agent degradation products. Environmental Health Perspectives. 1999;107(12):933-974.